Arrow™ EZ-IO™
Intraosseous Vascular Access System


The Arrow™ EZ-IO™ System is designed to provide vascular access in time-sensitive situations. Supported by over 15 years of clinical research and proven1 success, the EZ-IO™ System offers a fast2 and effective3 option for patients with difficult vascular access (DVA) with a well documented safety profile.
10
The speed you need
10
to achieve vascular access2*
3 seconds
for fluid and medication delivery to the heart through the proximal humerus3+
97
The success you depend on
97
first-attempt success rate4
Consistent
availability regardless of vein condition5
>1
The safety profile you trust
>1
serious complication rate6
Dependable
for critically ill patients in your care
To achieve rapid skill acquisition, fast access, success in resuscitation scenarios, and high first-attempt success rates7
![]()
Trusted for more than 15 years
>90 clinical trials
and case studies8
THOUSANDS of hospitals
in more than 80 countries8
65,000 clinicians trained globally
each year on average8
No charging necessary; no downtime or additional equipment needed.
• Designed for use in ground and air transport, hospital, and military environments.
• Fully sealed design for quick and easy cleaning.
• Green/red battery indicator light.
Secure catheter placement
Protects the insertion site
Recommend for use with all EZ-IO™ Needle Set insertions
Reinforced 90º angle to help prevent your line from kinking.
Support when needed
• Color-coded needle system enables quick selection and post-insertion identification.
• Diamond needle tip designed for precision performance.
• One needle with two insertion options allows for powered or manual (if necessary) insertion.
Promotes sharps safety.
Roll over to learn more


3
Non-shockable cardiac arrest
3
In drug treatment delay is associated with decreased survival9**
15
Acute severe hemorrhage cases
15
In drug treatment decreases survival10***
1
Sepsis cases
1
In antibiotic administration increases risk of mortality11
1
Stroke cases
1
In thrombolytic administration increases mortality12+
When every moment matters, it’s time to rethink DVA.
In a medical emergency, you may be ready but unfortunately some veins are not. The Arrow™ EZ-IO™ System supports your success by enabling fast2 access when you encounter patients with difficult vascular access.
The Arrow™ EZ-IO™ System provides immediate access via the intraosseous route8 for DVA cases that are emergent, urgent, or medically necessary, such as cardiac, shock, respiratory, and neurologic cases.
10
To achieve vascular access2++
3
For fluids and medication delivery to the heart through the proximal humerus3+++



Sepsis Kills a Patient in the U.S. Every 2.3 Minutes15

Overall, it kills more than AIDS and breast, colon, pancreatic, and prostate cancer combined16,17

But as many as 80% of sepsis deaths could be prevented with rapid diagnosis and treatment18

IV fluid resuscitation initiation administered within 30 minutes may decrease sepsis mortality rates and hospital length of stay19

Early antibiotics—ideally within the first hour—make a difference20
A study has shown that mortality increases 7.6% for every hour of delay in IV antibiotics18


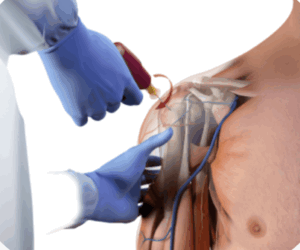
In patients with DVA, central venous catheter (CVC) access and peripheral IV (PIV) access can take longer and are less likely to be successful when compared with IO.32,33
Helping you fast-track patients with DVA
Provides a reliable bridge14 until longer-term vascular access can be established1
In a prospective, observational study on IO vs. CVC
during in-patient medical emergencies
HIGHER
First-pass success32IO: 90.3%
CVC: 37.5%
FASTER
Placement32++++IO: 1.2 min
CVC: 10.7 min
FEWER
Complications32+++++IO: 9.1%
CVC: 45.8%
Get in touch with our experts and learn how this device can support your services.

Arrow™ EZ-IO™ System
Rx Only.
The Arrow™EZ-IO™ Needle Set is Sterile, Single Use: Do not reuse, reprocess or re-sterilize. Reuse of device creates a potential risk of serious injury and/or infection which may lead to death. Refer to Instructions for Use for complete warnings, indications, contraindications, precautions, and potential complications.
Not all products shown on the website may be approved in all regulatory jurisdictions. Consult with your local Teleflex representative for details.
References
1. Dolister M, Miller S, Borron S, et al. J Vasc Access. 2013;14(3):216-224. Research sponsored by Teleflex Incorporated.; 2. Davidoff J, Fowler R, Gordon D, et al. JEMS. 2005;30(10):s20-s23. Research sponsored by Teleflex Incorporated.; 3. Montez DF, Puga T, Miller L, et al. Ann Emerg Med. 2015;66(4S):S47. Research sponsored by Teleflex Incorporated; 4. Brenner T, Bernhard M, Helm M, et al. Resuscitation. 2008;78(3):314-319.; 5. Neumar RW, Otto CW, Link MS, et al. Circulation. 2010;122(18 suppl 3):S729-S767.; 6. Teleflex Internal Data on File 2018.; 7. Levitan RM, Bortle CD, Snyder TA, et al. Ann Emerg Med. 2009;54(5):692-694.; 8. Teleflex Internal Data on File 2018.; 9. Donnino MW, Salciccioli JD, Howell MD, et al. BMJ. 2014;348:g3028.; 10. Gayet-Ageron A, Prieto-Merino D, Ker K, et al. Lancet. 2018;391(10116):125-132.; 11. Ferrer R, Martin-Loeches I, Phillips G, et al. Crit Care Med.2014;42(8):1749-1755.; 12. Fonarow GC, Smith EE, Saver JL, et al. Circulation. 2011;123(7):750-758.; 13. Davidoff J, et al. JEMS. 2005. Research sponsored by Teleflex Incorporated; 14. Leidel BA, Kirchhoff C, Bogner V, et al. Resuscitation. 2012;83(1):40-45.; 15. Marik PE. Ann Intensive Care. 2011; 16. HIV in the United States: at a glance. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Updated September 9, 2019. Accessed 23 Aug, 2023; 17. American Cancer Society Website. Cancer Facts & Figures 2019. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. Accessed 23 Aug, 2023; 18. Kumar et al. Crit Care Med. 2006; 19. Leisman D, et al. Annals of Emergency Medicine. 2016; 20. Dellinger, R. 2014; 21. Mozaffarian D, et al: Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015; 22. Clemency B, et al. Am J Emerg Med. 2016.; 23. Bramlett E, et al. Ann Emerg Med. 2016; 24. Based on Adult Proximal Humerus EZ-IO™ insertion data; 25. Compared to single lumen CVCs; 26. Hoskins SL, Zachariah BS, Copper N, Kramer GC. Comparison of intraosseous proximal humerus and sternal routes for drug delivery during CPR. Circulation 2007; 116:II_993. Research sponsored by Teleflex Incorporated. (preclinical study); 27. Hoskins SL, Nascimento P Jr., Lima RM, Espana-Tenorio, JM, Kramer GC. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation 2011; doi:10.1016/j.resuscitation.2011.07.041. Research sponsored by Teleflex Incorporated. (preclinical study); 28. Puga T, et al. Crit Care Med 2016. Research sponsored by Teleflex Incorporated. Based on healthy volunteer study.; 29. Teleflex Internal Data on File 2018; 30. Philbeck TE, et al. JEMS. 2010. Research sponsored by Teleflex Incorporated; 31. Cooper BR, Mahoney PF, Hodgetts TJ, Mellor A. Intra-osseous access (EZ-IO®) for resuscitation: UK military combat experience. J R Army Med Corps.2007;153(4):314-316; 32. Lee PJ, Lee C, Rattner P, et al. Crit Care Med.2015;43(6):1233-1238.; 33. Ross EM, et al. Am J Disaster Med. 2016
*Time to access is measured as insertion of the needle set through the bone cortex and into the intraosseous space.
†Based on adult proximal humerus study conducted in healthy individual.
**Epinephrine administered in hospital cardiac arrest.
***Analysis limited to administration of tranexamic acid.Data captured until 3 hours had elapsed.
+ Door-to-needle time for tPA.
+ IFU: The Arrow™ EZ-IO™ System is indicated for intraosseous access anytime in which vascular access is difficult to obtain in emergent, urgent or medically necessary cases
++Time to access is measured as insertion of the needle set through the bone cortex and into the intraosseous space.
+++ Based on adult proximal humerus study conducted in healthy individual.
++++Placement mean time is measured from package opening to confirmation of blood/marrow aspirate.
+++++All complications, including non-serious. Insertion sites were inspected 24 hours after initial placement.
Teleflex, Teleflex logo, Arrow, and EZ-IO are trademarks or registered trademarks of Teleflex Incorporated or its affiliates. All other trademarks or registered trademarks appearing on the web site are the property of their respective owners. MCI-2025-0082
The Arrow EZ-IO Needle Set is Sterile, Single Use: Do not reuse, reprocess or re-sterilise. Reuse of device creates a potential risk of serious injury and/or
infection which may lead to death. Refer to Instructions for Use for complete warnings, indications, contraindications, precautions, and potential
complications.
Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative